Brain modelling is a promising approach to investigate the structure and the dynamics of the brain, both in physiological and pathological conditions, and integrate knowledge cross various scales. They are employed to reconstruct neurobiological networks, replicate the brain’s activity, and anticipate its response to various stimuli. Eventually, once properly configured and validated, these models can generate their own ground-truth by binding the many parameters, provided by independent measurements and intrinsically prone to experimental error, into a coherent construct, and can be used to test various functional hypotheses.
The mouse brain is better characterized than the human brain, for which a greater lack of experimental data is accompanied by the inability to replicate most of the experimental sets used in laboratory animals. A translation from the mouse cerebellar model to a human model represents a step toward achieving what is referred to as Brain Digital Twin, opening new perspectives towards personalized and precision medicine
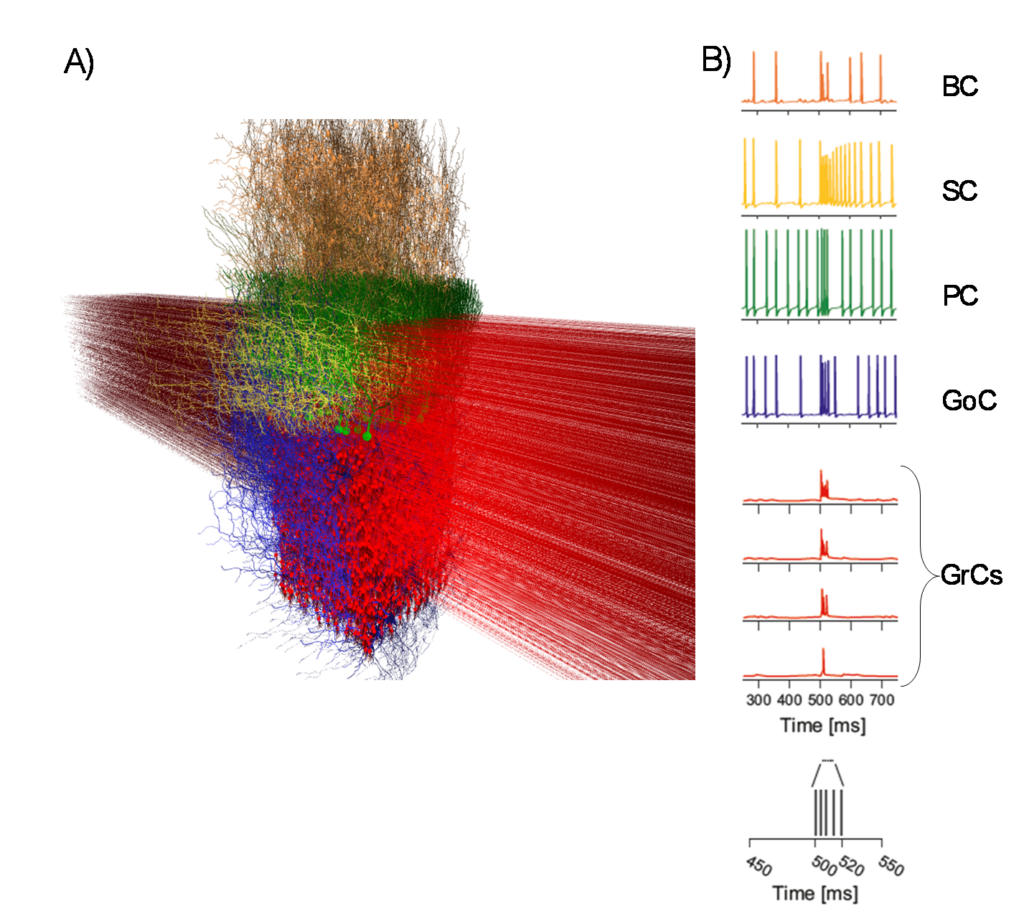
|
Cerebellar microcircuit model (A) Cerebellar model with cell morphologies in 3D space (granule cells – GrC, Golgi cells – GoC, Purkinje cells – PC, basket cells – BC and stellate cells – SC). (B) Activation of a vertical neuronal column in the cerebellar cortex. A whisker air-puff stimulus (a burst) is delivered. GrCs respond rapidly with a burst when at least 2 dendrites are activated. A GrC dense cluster is formed, and the signal propagates up through an ascending-axon bundle and transversally along a parallel-fiber beam. GoCs receive the signal both on basolateral and apical dendrites. PCs vertically on top of the active cluster are invested by GrC synaptic inputs. On-beam SCs and BCs receive signals through parallel-fiber synapses. The membrane potential traces (the whisker burst starts at 500 ms) are shown for each neuronal population. |
A PhD student involved in this research topic will have the opportunity to learn informatic programming in computational neuroscience, to reconstruct and simulate neural models, and to tune the models based on the experimental data.
References
- De Schepper R, Geminiani A, Masoli S, Rizza MF, Antonietti A, Casellato C*, D’Angelo E*. Model simulations unveil the structure-function-dynamics relationship of the cerebellar cortical microcircuit. NAT COMMS BIOL. 2022; doi: 10.1038/s42003-022-04213-y
- Geminiani A, Pedrocchi A, D’Angelo E, Casellato C. Response Dynamics in an Olivocerebellar Spiking Neural Network With Non-linear Neuron Properties. FRONT. COMPUT. NEUROSCI. 2019; 13:68; doi: 10.3389/fncom.2019.00068
- Geminiani A, Casellato C, Locatelli F, Prestori F, Pedrocchi A, D‘Angelo E. Complex dynamics in simplified neuronal models: reproducing Golgi cell electroresponsiveness. FRONT. NEUROINFORM. 2018; 12, 1–19; doi:10.3389/fninf.2018.00088
- D‘Angelo E, Antonietti A, Casali S, Casellato C, et al. Modelling the cerebellar microcircuit: new strategies for a long-standing issue. FRONT. CELL. NEUROSCI. 2016; 10:176; doi: 10.3389/fncel.2016.00176
- Masoli, M. F. Rizza, M. Sgritta, W. Van Geit, F. Schürmann, and E. D’Angelo, “Single neuron optimization as a basis for accurate biophysical modeling: The case of cerebellar granule cells,” Front Cell Neurosci, vol. 11, 2017, doi: 10.3389/fncel.2017.00071.
