With this technique cells recorded during an whole-cell patch-clamp experiment are loaded with a fluorescent dye. Flourescence allows to measure intracellular Ca2+ concentration changes using a CCD camera.
Intracellular calcium is related to many different physiological processes like neurotransmitters release, muscle contraction, ion channel gating, second messenger pathways, etc. For this reason, calcium quantification is performed in a variety of cellular studies to highlight molecular mechanism involved in different process.
Measuring intracellular calcium can be done using different techniques: the use of fluorescent calcium indicators (BAPTA based compounds) has become the most popular. These compounds are able to chelate Ca2+ causing a change in their fluorescence spectra.
We use Ca2+ imaging techniques to analyse Ca2+ ion dynamics in the dendrites of cerebellar granule cells and to investigate Ca2+ ion changes and their relationship with bidirectional long-term synaptic plasticity (LTD/LTP) at the mossy fibre-granule cell relay.
Example of dendritic specificity of Ca2+ signaling:
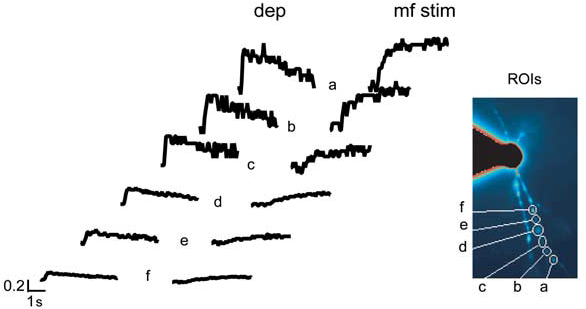
Ca2+ transients are measured at different dendritic positions (from a to f) and are induced either by a 200 ms depolarization (dep) from -70 to 0 mV or by high-frequency mf stimulation (mf stim; 1 s at 100 Hz) while holding the cell at -70 mV in the absence of extracellular Mg2+.
Relevant Pubblications:
-
David Gall, Francesca Prestori, Elisabetta Sola, Anna D’Errico, Celine Roussel, Lia Forti, Paola Rossi, Egidio D’Angelo (2005). Intracellular Calcium Regulation by Burst Discharge Determines Bidirectional Long-Term Synaptic Plasticity at the Cerebellum Input Stage. The Journal of Neuroscience, 25, pp. 4813-4822.
-
Anna D’Errico, Francesca Prestori and Egidio D’Angelo (2009). Differential induction of bidirectional long-term changes in neurotransmitter release by frequency-coded patterns at the cerebellar input. J Physiology, 2009, pp 1–15.
